A spooky Halloween story or chilling thriller film is incomplete without the appropriate array of creepy nighttime animal sounds. Unfortunately, many an evening stroll has been transformed into a terrifying, heart-pounding experience thanks to these bloodcurdling calls. In fact, we can encounter a variety of these sounds right here in the Pacific Northwest! And because we only fear what we don't understand, here is a brief guide to the spookiest of our local animal sounds to help you learn more about these relatively harmless creatures.
Barred Owl - Strix varia L: 21"
Common, year-round resident of the Puget Sound region. Often found in mixed coniferous-deciduous forests and seen during daytime hours near roosts. Calls include very loud hooting/barking, often described as monkey-like sounds. Individuals may also hiss or cackle.
Listen
Boreal Owl - Aegolius funereus L: 10"
Uncommonly seen inhabitant of montane forests in Eastern Washington. Found in mixed coniferous-deciduous northern forests. Call is a series of low, piping hoots that rise and fall in pitch and intensity. Also gives a low, nasal hooAh as well as short screeches.
Listen
Great Horned Owl - Bubo virginianus L: 22"
Fairly common and widespread, inhabiting a variety of habitats including forests, deserts, tundra, and urban areas. Often seen perched at dusk or mobbed by small birds in the daytime. Call is an iconic series of rhythmic hoots associated with most midwinter forest settings.
Listen
Western Screech-owl - Megascops kennicottii L: 8.5"
Common in wooded to sparsely wooded areas including urban parks, native woodland, and deserts. Generally roosts in very dense, dark areas and is difficult to see in the daytime. Call is a series of accelerating, abrupt whistled hoots that ends slightly lower in pitch.
Listen
(Can you hear the coyote in the background?)
Barn Owl - Tyto alba L: 16"
Extremely widespread species common to Western Washington. Prefers open grassland habitat and roosts in cavities or human-made structures. Populations may be in decline. These birds do not "hoot" like other owls. Calls generally only a shrieking screech. Very commonly thought to be a frightening, ghostly critter, but its silent flight and phenomenal hearing make it one of nature's best mouse-exterminators.
Listen
Northern Pygmy-owl - Glaucidium gnoma L: 6.75"
Widespread in coniferous forests throughout Washington. Some may migrate to lower elevations in winter months. Call is a regular, repeating series of tooting hoots. Listen for these distinctive calls at dawn and dusk.
Listen
Coyote - Canis latrans L: 45" H: 24"
Common throughout a variety of habitats, especially open areas and clearings. They have adapted well to the presence of humans and have expanded their ranges since the disappearance of the Gray Wolf. Sounds include yips, yaps, whines, barks and bone-chilling howls.
Listen (yips, yaps, barks, howls)
Listen ("moonlight howl")
Red Fox - Vulpes vulpes L: 40" H: 15"
Prefers brushy open habitats throughout Washington, but generally avoids densely forested areas and higher elevations. Varies in color from black to gray-brown. Sounds include barks, whines, wails and a fairly frightening scream (see video below).
Listen
-Robert Niese
Education and Outreach Coordinator
Friday, October 28, 2011
Friday, October 14, 2011
WOULD YOU BELIEVE A MILLION MEADOWHAWKS?
Yes, I said a million. Or perhaps 10 million or 100 million. Netta Smith and I visited Harney County in south-central Oregon on 24-26 September 2011 to look for birds, which as usual were in great numbers. But we were struck even more by the numbers of odonates. Rather than diversity, we found abundance. Two species were there in prodigious numbers like I have never seen before.
Striped Meadowhawks (Sympetrum pallipes) were the most abundant and ubiquitous. Our first stop near water was at a big pond on the east side of Hines. As soon as we stopped, we saw there were Sympetrum everywhere. On our first afternoon, the temperature reached 92° F, what I would call very hot. We stopped several times along highway 205 south of Burns and saw that the barbed-wire fences were covered with meadowhawks. They clearly chose to perch on the barbs rather than the wire between them, and in some places there was a dragonfly on just about every barb.
We stopped at Wrights Point, a long rocky ridge that extends out into the Blitzen River basin, and were flabbergasted at the numbers of Striped Meadowhawks there, flushing from the road and shrubs and dead tree branches. In addition, there were hundreds of Spotted Spreadwings (Lestes congener) in the shrubbery, only visible at closer range, although occasionally one would just fly across in front of me, perhaps flushed by one of the meadowhawks we were scaring up.
We got back into Burns at about 6:30 pm, and I immediately noticed dragonflies going past, a real flight. In all directions there were meadowhawks heading just a little east of south, right into the face of a moderate breeze. We estimated at least 10/minute passing in front of us, and we walked 50 yards to the grass behind the station and saw the same numbers. If they were flying over a broad front, there were hundreds/minute.
The sky was clouding up, and the flight fell off after about 15 minutes. At 6:45, there were still a few dragonflies passing, and there were many perched in the bushes. The flight seemed exactly like a migratory flight, yet the species is not known to be migratory, and I presume this was a local flight, perhaps individuals dispersing to find new breeding areas. I wondered if the high density of individuals would lead to such a flight.
The second day dawned cool, cloudy, and windy, and odonate activity fell dramatically. Nevertheless, the high temperature reached about 60° F., and we still saw meadowhawks and spreadwings wherever we stopped. It was warm enough that they were well able to fly as we flushed them, and many of them were perched in the same situations as on the preceding and following sunny day. The third day dawned clear and cold, but it warmed up rapidly to a high in the low 70s before we left the area to head back to Seattle.
On all three days, we stopped at additional oases of trees and shrubs in the midst of the vast prairie, shrub-steppe and wetland habitats, and every one of them was full of both Sympetrum and Lestes. As we walked along dirt paths, the meadowhawks rose one after another and fluttered off. We could look up a path and see them all over the sunlit ground. A diversion into the edge of any patch of woodland would reveal spreadwings all over the branches and twigs.
In several areas both species were perched on rock walls and sidewalks, the Lestes alongside the Sympetrum. As we flushed them, many of them ended up landing again near another, which in turn would flush, a chain reaction of fluttering. All the odonates were sexually mature; I never saw one that I could call immature.
Perhaps the third day was the most surprising. As we drove along a road through open fields just east of Burns at 11:30 am, temperature around 70° F, we started seeing Sympetrum flying across the road as we had on the evening of the first day. But they were in copulating pairs, not individuals! They were moving in the same direction as before, just east of south but still into a light breeze. I speculated that the wind direction was setting the flight direction, but who knows? This flight lasted the half hour that we were in that area and extended across at least a mile of road. Clearly thousands of individuals were participating.
As we drove into Burns at about noon, the flight was still going, patchy because of large buildings and areas of dense trees, but whenever we were in the open, there they were, crossing busy highways, all in exactly the same direction. Perhaps 10% of the individuals were single, but all the rest were in the wheel position. It was a fantastic sight. We saw no evidence of oviposition anywhere, nor did we see any sign of breeding in the spreadwings, yet I have no doubt that it took place somewhere.
We had to leave and started west on highway 20 toward Bend. There were still pairs going across the road up to about 10 miles west of Burns, then we saw no more but a few singles. We stopped in a rest area 16 miles west of Burns, and there were Striped Meadowhawks and Spotted Spreadwings all over the open grass, perched on the ground and in shrubs. Still the same species in abundance, but there was no mating, no flight. We stopped again at Hampton Station, much farther west, and again saw a few meadowhawks, but nothing in the air.
These observations were of the greatest interest to me in several ways. (1) I have never seen so many individuals of an odonate species spread over so much landscape. Underestimating all the time, I decided there was a minimum of 100 individuals/acre, and I think it was more like 1000/acre in many places. I thought the dragonflies were distributed over an area at least 50 x 30 miles, or 150 square miles. 150 square miles = 96,000 acres. At 100/acre, there were 9.6 million meadowhawks. Or perhaps there were 10x that many.
(2) Where did they all come from? Both species oviposit over dry land, then the basin fills with water during the winter rains, and the eggs hatch that spring, the larvae develop over the summer, and the adults are present in fall. So these individuals came from whatever wetlands filled during the winter of 2010-2011 and contained their eggs. 2011 has been an especially wet year, on the other hand, with Malheur Lake filling a huge area, so the eggs that were there produced a lot of larvae, thus the numbers of adults we saw.
But just a year ago, in spring 2010, there was almost no water in Malheur Lake, and the whole basin was dry, dry, dry. That would have provided much habitat for widespread oviposition of meadowhawks and spreadwings. But if it was dry, where did those adults come from? The puzzling aspect of this is that eggs had to be there in the first place from some previous successful year, and 2010 should have been a poor one for these species, with little water and thus few eggs hatching and relatively few adults. Is it possible that the eggs of these species (and of course other odonates) can lie dormant for multiple years?
(3) The meadowhawks were active in the sun at shockingly low temperatures on the third day. I saw several in flight when our car thermometer (known to be accurate within a degree or two) read 39° and 42° F at two stops. I have no idea how much their body temperature could have been raised by basking, but I almost never see odonates when it is colder than 55° F.
(4) We were really surprised to see no other species at all, except for the very occasional Blue-eyed Darner (Rhionaeschna multicolor); about a half dozen were seen over the three days, usually foraging in the lee of a tree grove. We checked several ponds and found nothing at all flying at them. How could one see a million odonates and see only three species?
As is so often the case in nature, observations generate more questions than answers.
Dennis Paulson
Labels:
dragonflies,
Malheur,
meadowhawks
Monday, October 3, 2011
MIGRATORY MONARCHS
The Monarch (Danaus plexippus) is surely the best known of North American butterflies. Occurring all across the continent, as well as on several other continents, this big orange butterfly with a vivid black-lined pattern delights all who see it. Like the swallowtails, it is the epitome of butterflyhood, bouncing across meadows and sipping nectar from a wide variety of flowers.
At this time of year, Monarchs all across North America head south for the winter. The vast majority acome from the eastern two-thirds of the continent. They fly south in trickles, then streams, then rivers converging on just a few areas in the mountains of central Mexico, wintering grounds not discovered by scientists until 1975. Many fewer (about 5% of the total population) head for the Pacific coast to winter in a few coastal groves in California.
Although many insects are migratory, this one is the most noteworthy in the US because of its large size, bright coloration, and great numbers of individuals on the move at once.
If you live near milkweed plants (Asclepias species), you may be very familiar with Monarchs. The beautiful flowers of milkweeds give no hint of their toxic nature. The plants have a milky sap full of cardiac glycosides, chemicals that affect the sodium-potassium pump in vertebrate cell membranes and can cause tachycardia and ventricular fibrillation, both very stressful to the human heart. The sap is also viscous and bitter tasting and caustic to our mucous membranes.
Monarchs breed on milkweeds, and their caterpillars are among the small number of insects that have evolved a resistance to the harmful effects of milkweed sap. They munch it with no worries throughout their development. The sap confers unpalatability and distastefulness on the larvae, and their bright aposematic coloration serves as a warning flag to potential predators.
The poisonous qualities of the milkweed are carried through metamorphosis, and the butterflies are also distasteful, unpalatable, and probably warningly colored. They are left alone by vertebrate predators, although invertebrates such as dragonflies can handle them.
Monarchs are large and conspicuous, not especially fast flying, and if they weren't distasteful they would probably be the object of much predation during their migrations and certainly on their communal roosts. In fact, they probably roost communally to concentrate the warning to predators that they are not to be touched.
Monarchs are nowhere nearly as common in the West as in the East, and in Washington state they are downright uncommon. It's always thrilling to see an adult or larval Monarch in the Columbia Basin, where they are associated with the big milkweed Asclepias speciosa. Presumably our Monarchs head south to the California roosts.
After overwintering, Monarchs take off for the North. They move back into southern US and breed where they stop. Their larvae develop quickly in the spring and, as soon as they are out of the pupa, the adults head north like the previous generation. They are one-way migrants, and they will stop somewhere in the northern US or even southern Canada to breed. There may be as many as three northbound migrant generations, before their offspring head off in the other direction, back to the wintering ground.
Dennis Paulson
Labels:
butterflies,
migration,
monarch
Tuesday, September 20, 2011
TIGERS OF THE SAND
Go to a sandy beach or an open patch of sandy soil just about anywhere in the summer and you are likely to see tiger beetles. You will at first see these diurnal insects running ahead of you. Their long, slender legs propel them over the ground at amazing speeds. If you get too close, they jump into the air, open their elytra (wing covers), and quickly fly away. They usually land nearby, and you may be able to follow individual beetles until one lets you get close enough for prolonged observation.
But look quickly, as they are likely to run and stop, run and stop as they hunt for prey. They have exceptionally long, sharply pointed, tooth-lined mandibles, with which they capture other insects and spiders. They make short work of their prey and move on to hunt again. Their vision is superb, both to find prey and avoid predators.
Even though they are alert and fast, they do have predators, including birds, lizards, and robber flies. Birds such as kestrels and flycatchers capture them in the air, shrikes on the ground. As well as their obvious adaptations, some tiger beetles secrete defensive chemicals that presumably protect them from some predators.
The cuticle of tiger beetles is somewhat iridescent, and although the majority of species are sort of a bronzy brown, many of them are brightly colored, usually green but sometimes purple or blue. Some species are polymorphic, coming in two or more of these colors. The undersides are often more metallic than the upper surfaces. Some species have red abdomens that show up when they fly. Most tiger beetles have a characteristic pattern of spots and lines on their elytra, and variations on that pattern are often what define different species.
Unlike many insects, when tiger beetles mate they both face the same way, so they can continue to run across the ground (but not to fly) when the male is perched on the female's back. This lessens the likelihood of predation when they are in this vulnerable state. A male may remain on the female's back after copulation to keep other males from mating with her. Females lay their eggs, one at a time, into the soil in places appropriate for the larva.

Tiger beetle larvae are just as predacious as the adults, but we don't see them at work. They live in burrows in the sand, covered except for a hard head capsule and a pair of mandibles. When another insect comes too close, they reach up from the surface and grab it, then pull it down into their burrow, to which they are anchored by hooks on top of the fifth abdominal segment. They have been known to capture dragonflies of much larger size that had the bad luck to land right at the mouth of a burrow.
There are 17 species of tiger beetles known from Washington state, all in the day-active, brightly colored genus Cicindela except for two nocturnal black species of Omus. Few of the species are statewide; most have limited ranges on one side of the Cascades, up in the mountains, or along the coast or big rivers.
Fortunately for aficionados of this group, there are two fine books available:
A Field Guide to the Tiger Beetles of the United States and Canada, by David L. Pearson, C. Barry Knisley, and Charles J. Kazilek, Oxford University Press, 2006.
Tiger Beetles: The Evolution, Ecology, and Diversity of the Cicindelids, by David L. Pearson and Alfried P. Vogler, Cornell University Press, 2001.
Fortunately for aficionados of this group, there are two fine books available:
A Field Guide to the Tiger Beetles of the United States and Canada, by David L. Pearson, C. Barry Knisley, and Charles J. Kazilek, Oxford University Press, 2006.
Tiger Beetles: The Evolution, Ecology, and Diversity of the Cicindelids, by David L. Pearson and Alfried P. Vogler, Cornell University Press, 2001.
Dennis Paulson
Labels:
beetles,
predation,
tiger beetles
Wednesday, August 3, 2011
A LOT OF DRAGONFLIES
The Spiny Baskettail (Epitheca spinigera) is a dragonfly of the emerald family (Corduliidae) that occurs all the way across North America from Atlantic to Pacific. It is common in southern Canada and the northern tier of US states. It breeds in lakes, and apparently it can become very common in its optimal habitats.
It shares with numerous other species of Odonata the life-history trait of overwintering in the final larval instar and thus being ready to emerge as soon as its aquatic habitat warms sufficiently in spring. When this happens, apparently most of the larvae in the lake are ready to emerge at about the same time, so there are massive emergences over a period of a few days. I have yet to witness one of these (the larvae actually crawling out of the water, splitting their skins, and the adult that emerges flying away), but I did see the aftermath.
These scenes greeted us one sunny morning in June in southwestern Manitoba. Spiny Baskettails, hundreds and hundreds of them of both sexes, were hanging from every bare twig for several hundred meters along the entry road to Lake Audy in Riding Mountain National Park. The location is outside the Northwest, but the species occurs in the Northwest, so I consider it fair game.
Several things were of interest, besides the sheer staggering numbers of them. Very few were flying around, yet I would have thought there would be many prey insects in the air on this nice warm morning. Secondly, not a single one was resting on a leaf; every individual was hanging from a bare twig, even though it meant that when one was dislodged, it wasn’t that easy to find a new perching spot.
Several times we saw one try to land on the abdomen of a perched individual, but they were always shaken or buzzed off. All individuals were obviously immature, with reddish eyes. During sexual maturation, the eyes become glowing blue-green.
The sexes can readily be distinguished with a good view. Males have a slightly more slender abdomen base, with secondary genitalia projecting downward near the base, and three terminal appendages (two dorsal and one ventral). Females lack the bump at the base and have only the two dorsal appendages at the tip.
Mother Nature was showing off her profligacy in a big way here in an aspen woodland at the southern edge of the Canadian boreal forest.
Dennis Paulson
Labels:
dragonflies,
emergence,
seasonality
Thursday, June 23, 2011
NAKED IN THE OCEAN
It’s time to home in on the extreme low tides we get in our local marine environment during daylight hours in the summer. Get to the coast an hour before the low and spend a couple of hours looking in the shallow water and especially in pools left by the receding tide. If the pool is rich enough, just sit down and enjoy watching the invertebrates and fish, if there are any, go about their business.
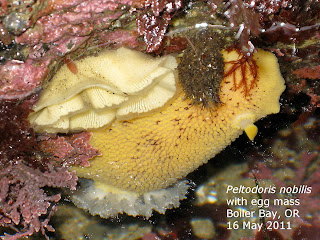
But don't think you have to shed your clothes; the nudity here involves the animals you are watching. Among the animals that inhabit such places are the nudibranchs (= naked gills). These are mollusks that appear to lack shells, something like seagoing slugs; many have prominent external gills. Like slugs, they are members of the class Gastropoda, which includes the snails. Having dispensed with a shell gives the nudibranchs both constraints and opportunities.
The primary constraint, of course, would seem to be a much greater risk of predation without a hard shell into which to retire when disturbed. Along with this is the risk of desiccation when exposed at low tides, so you won’t find many nudibranchs sitting around on exposed rocks like you do some of the snails.
These are mostly small animals, ranging from 1 cm (Rostanga) up to 20 cm (Peltodoris), but because of their conspicuousness and beauty, they are more admired and sought by tidepoolers than some of the other invertebrates that share their habitat.
Prominent anatomical features are two tentacle-like structures toward the front that are called rhizophores and are probably chemical detectors. Toward the rear there may be a ring of gills. And projections all along the upper surface are cerata, the bright colors of which are probably involved in predator deterrence, a warning coloration that goes along with distastefulness.
The lack of a shell has provided selective pressure for nudibranchs to develop other ways of protecting themselves from predators, and one of the most effective is the ability to secrete chemicals, including sulfuric acid in some cases, that make them inedible. They are surprisingly predator-free, and fish have been seen to spit them out. Anything as conspicuous as some of these are would seem to be predator resistant!
One of the opportunities for such animals comes in the form of being free to be predators themselves. They often specialize in animals that aren’t eaten by many other predators, thus assuring them a reliable food supply. Most of their prey items are sessile, fixed to the rocks on which they crawl, but a few are able to capture motile animals as well.
Acanthodoris eats colonial ascidians (tunicates) and bryozoans. Triopha and Janolus eat arborescent (branched) bryozoans. Doris and Peltodoris eat sponges, especially the crumb-of-bread sponge (Halichondra), and how they avoid being pierced by the sponge spicules hasn’t been determined. These two species are called sea lemons. The tiny Rostanga eats red sponges, on which it may be perfectly camouflaged. Hermissenda eats hydroids and incorporates their toxic nematocysts into its cerata, thus affording it protection from predators. Dirona eats everything, including not only the same prey as the others but also snails and crustaceans.
The species shown here were found at two localities on the north coast of Oregon in May. All are common in Pacific Northwest waters.
Dennis Paulson
Labels:
mollusks,
nudibranchs,
tidepools
Thursday, June 2, 2011
THE CHORUS OF THE CHORUS FROGS
Early every year in the Pacific Northwest a familiar sound rings out, telling us it is spring whether it seems that way or not. this is the “song” or advertisement call of the male Pacific Chorus Frog, Pseudacris regilla.
Most people recognize the call immediately as the generic frog call of movies in decades past. As this is a common frog (or at least used to be) in Hollywood, California, its call was incorporated into many a movie that needed frog calls as ambience. In fact the “ribbit, ribbit” sound has become the stereotype of frog calls.
These frogs come out of their winter dormancy very early in spring, when, to paraphrase Robert Burns, a young frog’s fancy lightly turns to thoughts of love. The only way a frog has to express itself in such a situation is to call . . . . and call . . . . and call. And that they do, with surprising strength.
When a male frog feels these stirrings, he heads for the nearest pond or marsh, usually in the evening but sometimes even during the day. On arrival, he jumps in the water and swims to what he considers a good position. Only the frog knows why it is a good position, but it probably provides a place to hold onto the vegetation and a place where he can be easily seen as well as heard. Interestingly, it is often the same male that calls first each evening.
He begins to call: ribbit, ribbit, ribbit, a creaky two syllables that carries at least a hundred yards or more on a quiet night. Another frog heads for the pond, either because hopping downhill in a moist environment will lead to water or because it homes in on the first frog. The second frog begins to call, perfectly insinuating its calls between those of the first. The two may sound quite different, so we hear ribbit, rabbit, ribbit, rabbit, ribbit, rabbit.
A third male begins to call, amazingly also able to insert its calls into the soundscape so they can be heard as distinct: ribbit, rabbit, robbit, ribbit, rabbit, robbit . . . the pace is speeding up, and there is no room for a fourth frog, but that one calls anyway. As the chorus swells, the individual voices become less apparent, even though the structure may still be there, but a female approaching the pond can easily distinguish the individual voices.
Females apparently choose males based on the vigor of their songs, and as the evening progresses, more and more males acquire a mate. The male clasps the female and stays with her while she looks for a place to lay her eggs. She finds such a place, lays a cluster of eggs, and the male fertilizes them.
The eggs hatch in a few days, and the tadpoles grow quickly on a diet of plant matter. After a few months, they finally absorb their tails and grow a set of limbs and are then ready to leave the pond. If they survive the year, they will return the following spring, and the pond will resound again with the chorus frog chorus.
Dennis Paulson
Subscribe to:
Posts (Atom)